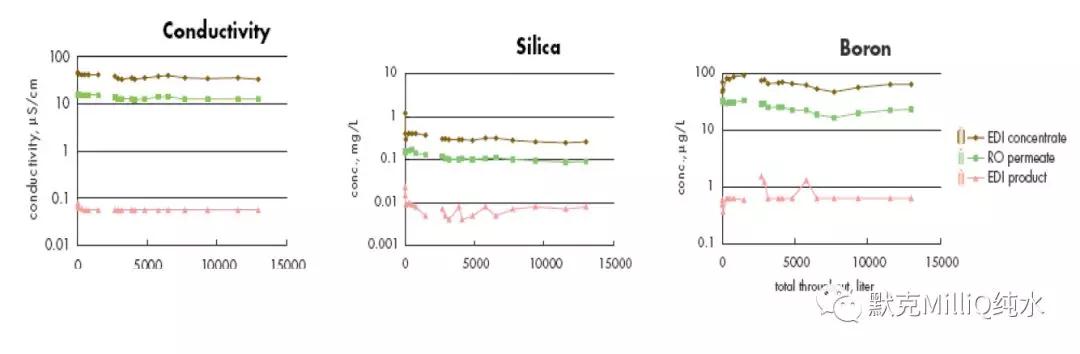
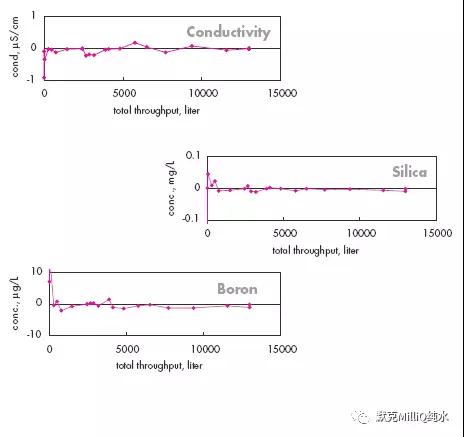
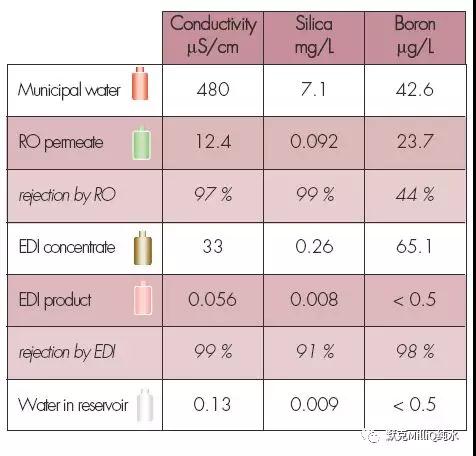
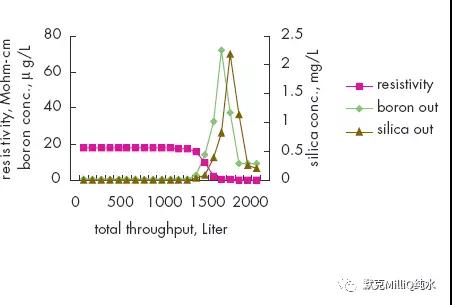
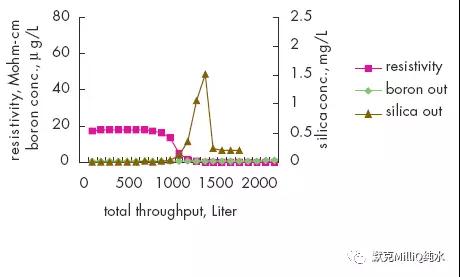
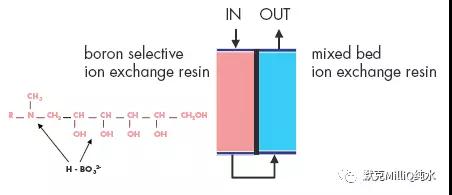
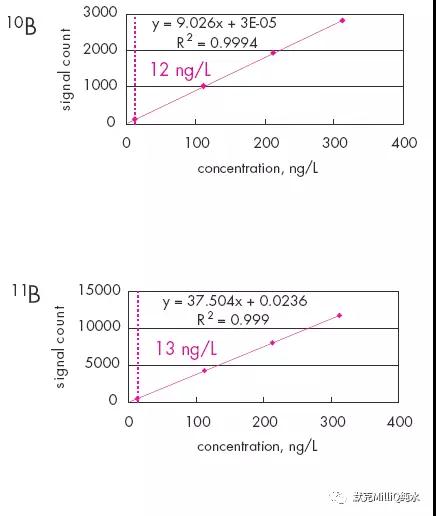
图10 终端纯化结果:痕量的硼分析
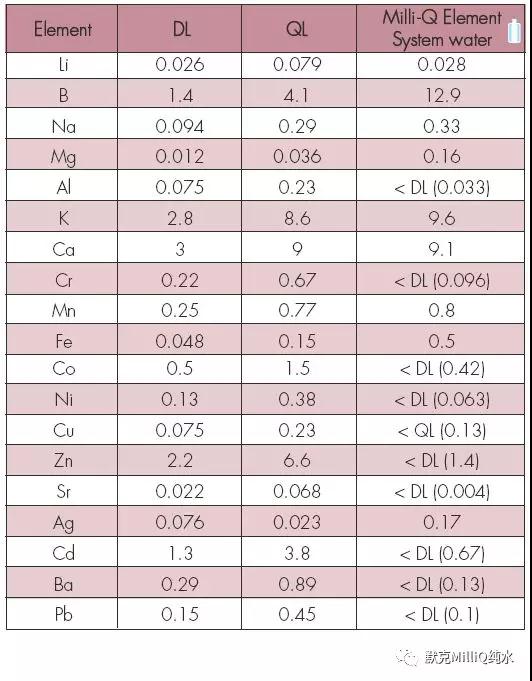
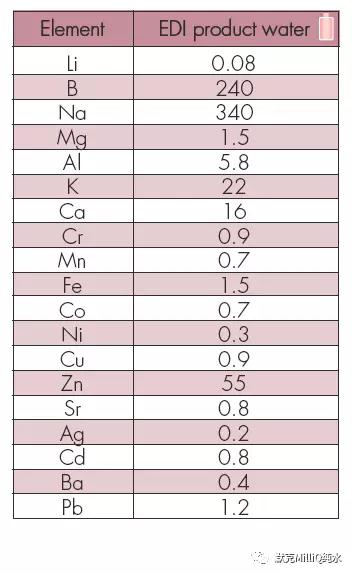
表5 ICP-MS 对Milli-Q Element 水进行痕量分析——所有的值均为ppb 级(ng/L)
监测与量化的计算极限很低,能够用于ng /L 水平分析。 对于11B 测量极限为1.4 ng /L,量化极限为4.1 ng / L。
使用分光光度法测定的硅元素的浓度极限为1 ppb 以下。
此外,这个系统可实现大多数元素在ppt 水平的水质;实际上,许多元素的含量在测量极限之下。对这个研究中的所有元素,终端精制元件都表现了较高的截留率,达到ppt 级的水质。
这个用来进行痕量分析的纯化系统,在以前的文章中被详细描述15。
结论
在进行ppt 级的痕量分析中,超纯水被用于制备空白液、标准液及样品稀释。利用某种特性和[配置可以达到大多数元素的检测限。然而,向硼和硅这样的弱电离离子,他们不影响电阻率,所以不容易被用户发觉。有待于研究如何降低这些弱电离离子的穿透能力。另外,超纯水中痕量硅元素的纯化和灵敏度的研究仍在进行中。本文中描述的水纯化链中确保了稳定、最小的元素污染。EDI 模块保证了较好的离子平衡且在使用寿命内无需离子再生。
反渗透技术结合连续电流去离子技术对包括硼离子在内的所有离子都显示了较高的离子截留率和稳定性。在最后的水纯化单元,通过使用特殊设计的硼去除纯化柱可以降低早期的硼穿透现象。这些技术的结合保证达到适合于ppt 级、次ppt 级痕量分析对超纯水的要求。
参考文献
1 Wibowo, J.; Shadman, F.; Blackmord,D.; ÒMeasuring and removing dissolved and colloidal
silica inultrapure waterÓ, Micro 15 (5) pp 22-25 (1997)
2 Chu, T.; M. K., Balazs;“Determination of Total Silica at PPB Levels in High-Purity Water by
Three Different Analytical Techniques”,Ultrapure Water,11 (1) pp56-60 (1994)
3 Malhotra, S.; Chan, O.; Chu, T.;Fuckso, A.; “Correlation of Boron Breakthrough Versus
Resistivity and Dissolved Silica inRO/DI System”, Ultrapure Water May/June 1996 pp 22-25.
(1996)
4 Rychen, P.; Druges, M.; Mar_ ais, N.; “BoronBehavior in High-Purity Water Plants with
RO/MB Systems and RO/EDI System”, Ultrapure Water 15(10) pp22-32 (1998)
5 Ooi, T.; Uraguchi, D.; Kagoshima, N.;Maruoka, K.; “Hypercoordination of Boron and
Aluminum: Synthetic Utility asChelating Lewis Acids”, Journal of the American Chemical
Society 120 (21) pp 5327-5328(1998)
6 Bi_ ak, N; Filiz, B.; “Sorbitol-modified poly(N-glycidylstyrene sulfonamide) for removal of
boron” Journal of Applied Polymer Science 68(13) pp2113-2119 (1998)
7 Ferrier, R.J.; “CarbohydrateBoronates” in Carbohydrate Chemistry : monosaccharides,
disaccharides, and specificoligosaccharides pp 31-79 (1991)
8 Mardan, A.; “Enrichment of boron-10by inverse-frontal chromatography using quaternized
4-vinylpyridine-divinylbenzeneanion-exchange resin” Separation Science and Technology 32 (13)
pp 2115-2125 (1997)
9 Sahin, S.; “Mathematical Model for Adsorptionof Boric Acid on a Boron-Specific Ion
Exchanger”, Bulletin of the Chemical Society ofJapan 69 (7) pp 1917-1920 (1996)
10 Xiao, Y.K.; Vocke, Jr., R.D.;Swihart, G.H.; Xiao, Y.; “Boron Volatilization and Its Isotope
Fractionation during Evaporation ofBoron Solution”, Analytical Chemistry 69 (24) pp 5203-5207
(1997)
11 Stewart, B.M.; Darbouret D.“Advancements in the production of ultrapure water for ICP-MS
metals analysis”, AmericanLaboratory News 30 (9), pp 36-38 (April 1998)
12 Darbouret, D.; Kano, I.; Youf, E.;Stewart, B.M. “Optimizing storage of High Purity Water”
The R&D Notebook, A publication ofthe Laboratory Water Division of Millipore: R&D Notebook
RD001, (1998)
13 Lapid, J.;Farhi, S.; Koresh, Y.“Spectrofluorometric determination of Boron with chromotropic
acid”, Analytical letters 9 (4), pp 355-360,(1976)
14 Motomizu, S.; Oshima, M.; Toei, K.“Fluorometric determination of Boron with chromotropic
acid by continuous flow system” Bunseki Kagaku 32 pp 458-463,(1983)
15 Darbouret, D.; Kano, I.; “UltrapureWater for Elemental Analysis down to ppt levels”,The
R&D Notebook, a publication of theLaboratory Water Division of Millipore. RDOO2, (1999)