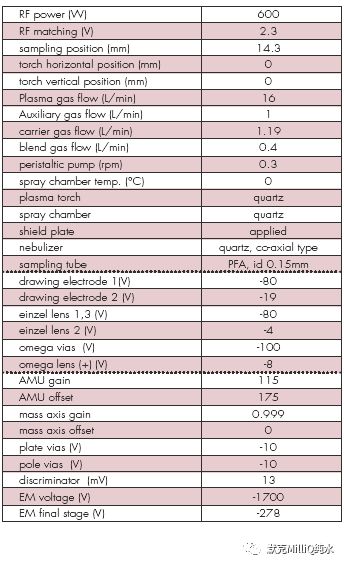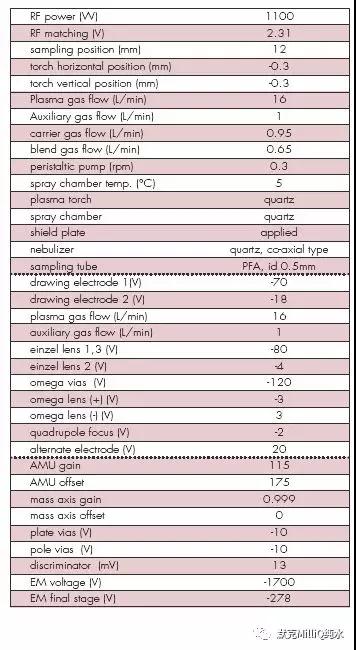
表2 用HP4500 ICP-MS 进行多元素分析
硼标准液从Kanto Kagaku 公司购买。
0.5mMPFA 进样管直接连接喷雾器,通过自吸来进样。 制备标准溶液和样品时避免溶液和外部环境之间的接触,用带有聚乙烯瓶盖的样品瓶盛装,防止进入分析器的样品被微粒污染。
作为标准样的多元素溶液购自SPEX(Cat. N XSTC-331).它包含28 种元素,用来绘制标准曲线。
超纯级硝酸(购自 Kanto Kagaku )用于制备酸化标准液和稀释液。
标准曲线使用标准加入法绘制。检出限( DL )3 倍于十次空白的标准偏差( Milli-Q SP ICPMS 水,Nihon Millipore 有限公司),定量检测极限( ( QL )是检出限的3.33 倍(或者10δ )。
由于硼元素易残留在进样器内(Memory Effect),因此在痕量分析中难于检测,需要用过氧化氢溶液清洗进样器、雾化器、雾化室和离子炬(plasma torch)以消除残余的硼。 在重新设定ICP-MS 之前,记录10B 和11B 指定m/z 的稳定性。测量要从低浓度到高浓度。建议先测待测样品,最后测标准样绘制标准曲线:这是因为标准样浓度较大,残留的硼元素更易干扰测量。
电导率和电阻率测量
水的电导率和电阻率可以表征水的离子污染程度。内置电导率和电阻率测量设备用来监控这些数值。
未完待续。
参考文献
1 Wibowo, J.; Shadman, F.; Blackmord,D.; ÒMeasuring and removing dissolved and colloidal
silica inultrapure waterÓ, Micro 15 (5) pp 22-25 (1997)
2 Chu, T.; M. K., Balazs;“Determination of Total Silica at PPB Levels in High-Purity Water by
Three Different Analytical Techniques”,Ultrapure Water,11 (1) pp56-60 (1994)
3 Malhotra, S.; Chan, O.; Chu, T.;Fuckso, A.; “Correlation of Boron Breakthrough Versus
Resistivity and Dissolved Silica inRO/DI System”, Ultrapure Water May/June 1996 pp 22-25.
(1996)
4 Rychen, P.; Druges, M.; Mar_ ais, N.; “BoronBehavior in High-Purity Water Plants with
RO/MB Systems and RO/EDI System”, Ultrapure Water 15(10) pp22-32 (1998)
5 Ooi, T.; Uraguchi, D.; Kagoshima, N.;Maruoka, K.; “Hypercoordination of Boron and
Aluminum: Synthetic Utility asChelating Lewis Acids”, Journal of the American Chemical
Society 120 (21) pp 5327-5328(1998)
6 Bi_ ak, N; Filiz, B.; “Sorbitol-modified poly(N-glycidylstyrene sulfonamide) for removal of
boron” Journal of Applied Polymer Science 68(13) pp2113-2119 (1998)
7 Ferrier, R.J.; “CarbohydrateBoronates” in Carbohydrate Chemistry : monosaccharides,
disaccharides, and specificoligosaccharides pp 31-79 (1991)
8 Mardan, A.; “Enrichment of boron-10by inverse-frontal chromatography using quaternized
4-vinylpyridine-divinylbenzeneanion-exchange resin” Separation Science and Technology 32 (13)
pp 2115-2125 (1997)
9 Sahin, S.; “Mathematical Model for Adsorptionof Boric Acid on a Boron-Specific Ion
Exchanger”, Bulletin of the Chemical Society ofJapan 69 (7) pp 1917-1920 (1996)
10 Xiao, Y.K.; Vocke, Jr., R.D.;Swihart, G.H.; Xiao, Y.; “Boron Volatilization and Its Isotope
Fractionation during Evaporation ofBoron Solution”, Analytical Chemistry 69 (24) pp 5203-5207
(1997)
11 Stewart, B.M.; Darbouret D.“Advancements in the production of ultrapure water for ICP-MS
metals analysis”, AmericanLaboratory News 30 (9), pp 36-38 (April 1998)
12 Darbouret, D.; Kano, I.; Youf, E.;Stewart, B.M. “Optimizing storage of High Purity Water”
The R&D Notebook, A publication ofthe Laboratory Water Division of Millipore: R&D Notebook
RD001, (1998)
13 Lapid, J.;Farhi, S.; Koresh, Y.“Spectrofluorometric determination of Boron with chromotropic
acid”, Analytical letters 9 (4), pp 355-360,(1976)
14 Motomizu, S.; Oshima, M.; Toei, K.“Fluorometric determination of Boron with chromotropic
acid by continuous flow system” Bunseki Kagaku 32 pp 458-463,(1983)
15 Darbouret, D.; Kano, I.; “UltrapureWater for Elemental Analysis down to ppt levels”,The
R&D Notebook, a publication of theLaboratory Water Division of Millipore. RDOO2, (1999)